Could you please explain density?
What is it? What is the concept of D = M over V?
Density
is how much mass a material has for a given volume.
- A measure of the compactness of the molecules of a material. The closer the particle are to one another the higher the density of the material. The mass per unit of volume of a material.
D = m/v
D = density
m = mass
v = volume
Mass = The amount of matter inside an object. It does not change based on gravity. It is usually measured in grams or kgrams.
Volume = the amount of space an object occupies. Usually measured in L, ml, or cm3 using a graduated cylinder.
Think about a
sponge. Most artificial sponges today are made of a foamed plastic.
Assume you have a one pound sponge. If you melt it down to a plastic
soup with all the bubbles gone, it will be much smaller, but it will
still weigh one pound. It is now denser.
We use water as sort of a
standard for density. We say water has a density of 1. If something
weighs twice as much as the same volume of water we say its density is
two.
- This is really handy for figuring out all sorts of things like if something will float or not.
Ice is ~12% less dense than water so ice floats. Water gets bigger when it freezes so ice is less dense than water. It's a good thing too since life probably would not exist if that were not the case. A U.S. air craft carrier weighs more than 200,000,000 pounds, but I know its density is still less than one. I know this without even asking anyone since I have seen them floating.
An object that is very dense is usually very heavy for its size.
- For example, a bowling ball is more dense than a beach ball. Sometimes, the density of an object can change, like when you pack a snowball - the snow starts out light and fluffy and then you compress it to a higher density.
Archimedes' Principle
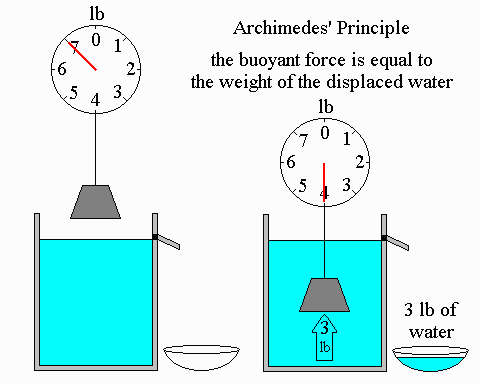
-
If the weight of the water displaced is less than the weight of the object, the object will sink
-
Otherwise the object will float, with the weight of the water displaced equal to the weight of the object.
Archimedes' Principle explains why steel ships float
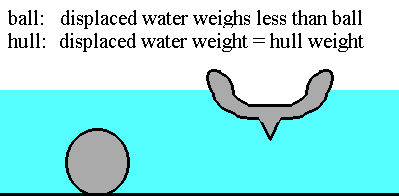
AFTER WATCHING THE ARCHIMEDES' PRINCIPLE VIDEO -
- CLICK ON THE "THINK" BUTTON RIGHT COLUMN.
- ANSWER THE QUESTIONS on a piece of notebook paper & turn into the drawer.












