- So waves are everywhere. But what makes a wave a wave?
- What characteristics, properties, or behaviors are shared by the phenomena that we typically characterize as being a wave?
- How can waves be described in a manner that allows us to understand their basic nature and qualities?
A wave can be described as a disturbance that travels through a medium from one location to another location.
Consider a slinky wave as an example of a wave.
- When the slinky is stretched from end to end and is held at rest, it assumes a natural position known as the equilibrium or rest position. The coils of the slinky naturally assume this position, spaced equally far apart. To introduce a wave into the slinky, the first particle is displaced or moved from its equilibrium or rest position. The particle might be moved upwards or downwards, forwards or backwards; but once moved, it is returned to its original equilibrium or rest position. The act of moving the first coil of the slinky in a given direction and then returning it to its equilibrium position creates a disturbance in the slinky. We can then observe this disturbance moving through the slinky from one end to the other. If the first coil of the slinky is given a single back-and-forth vibration, then we call the observed motion of the disturbance through the slinky a slinky pulse. A pulse is a single disturbance moving through a medium from one location to another location. However, if the first coil of the slinky is continuously and periodically vibrated in a back-and-forth manner, we would observe a repeating disturbance moving within the slinky that endures over some prolonged period of time. The repeating and periodic disturbance that moves through a medium from one location to another is referred to as a wave.
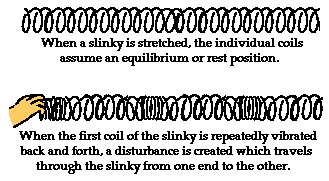
But what is meant by the word medium? A medium is a substance or material that carries the wave. You have perhaps heard of the phrase news media. The news media refers to the various institutions (newspaper offices, television stations, radio stations, etc.) within our society that carry the news from one location to another. The news moves through the media. The media doesn't make the news and the media isn't the same as the news. The news media is merely the thing that carries the news from its source to various locations. In a similar manner, a wave medium is the substance that carries a wave (or disturbance) from one location to another. The wave medium is not the wave and it doesn't make the wave; it merely carries or transports the wave from its source to other locations. In the case of our slinky wave, the medium through that the wave travels is the slinky coils. In the case of a water wave in the ocean, the medium through which the wave travels is the ocean water. In the case of a sound wave moving from the church choir to the pews, the medium through which the sound wave travels is the air in the room. And in the case of the stadium wave, the medium through which the stadium wave travels is the fans that are in the stadium.
To fully understand the nature of a wave, it is important to consider the medium as a collection of interacting particles. In other words, the medium is composed of parts that are capable of interacting with each other. The interactions of one particle of the medium with the next adjacent particle allow the disturbance to travel through the medium. In the case of the slinky wave, the particles or interacting parts of the medium are the individual coils of the slinky. In the case of a sound wave in air, the particles or interacting parts of the medium are the individual molecules of air. And in the case of a stadium wave, the particles or interacting parts of the medium are the fans in the stadium.
Consider the presence of a wave in a slinky. The first coil becomes disturbed and begins to push or pull on the second coil; this push or pull on the second 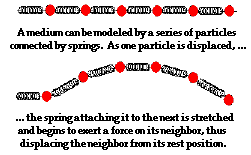 coil will displace the second coil from its equilibrium position. As the second coil becomes displaced, it begins to push or pull on the third coil; the push or pull on the third coil displaces it from its equilibrium position. As the third coil becomes displaced, it begins to push or pull on the fourth coil. This process continues in consecutive fashion, with each individual particle acting to displace the adjacent particle. Subsequently, the disturbance travels through the medium. The medium can be pictured as a series of particles connected by springs. As one particle moves, the spring connecting it to the next particle begins to stretch and apply a force to its adjacent neighbor. As this neighbor begins to move, the spring attaching this neighbor to its neighbor begins to stretch and apply a force on its adjacent neighbor.
coil will displace the second coil from its equilibrium position. As the second coil becomes displaced, it begins to push or pull on the third coil; the push or pull on the third coil displaces it from its equilibrium position. As the third coil becomes displaced, it begins to push or pull on the fourth coil. This process continues in consecutive fashion, with each individual particle acting to displace the adjacent particle. Subsequently, the disturbance travels through the medium. The medium can be pictured as a series of particles connected by springs. As one particle moves, the spring connecting it to the next particle begins to stretch and apply a force to its adjacent neighbor. As this neighbor begins to move, the spring attaching this neighbor to its neighbor begins to stretch and apply a force on its adjacent neighbor.
 coil will displace the second coil from its equilibrium position. As the second coil becomes displaced, it begins to push or pull on the third coil; the push or pull on the third coil displaces it from its equilibrium position. As the third coil becomes displaced, it begins to push or pull on the fourth coil. This process continues in consecutive fashion, with each individual particle acting to displace the adjacent particle. Subsequently, the disturbance travels through the medium. The medium can be pictured as a series of particles connected by springs. As one particle moves, the spring connecting it to the next particle begins to stretch and apply a force to its adjacent neighbor. As this neighbor begins to move, the spring attaching this neighbor to its neighbor begins to stretch and apply a force on its adjacent neighbor.
coil will displace the second coil from its equilibrium position. As the second coil becomes displaced, it begins to push or pull on the third coil; the push or pull on the third coil displaces it from its equilibrium position. As the third coil becomes displaced, it begins to push or pull on the fourth coil. This process continues in consecutive fashion, with each individual particle acting to displace the adjacent particle. Subsequently, the disturbance travels through the medium. The medium can be pictured as a series of particles connected by springs. As one particle moves, the spring connecting it to the next particle begins to stretch and apply a force to its adjacent neighbor. As this neighbor begins to move, the spring attaching this neighbor to its neighbor begins to stretch and apply a force on its adjacent neighbor.
A Wave Transports Energy and Not Matter
When a wave is present in a medium (that is, when there is a disturbance moving through a medium), the individual particles of the medium are only temporarily displaced from their rest position. There is always a force acting upon the particles that restores them to their original position. In a slinky wave, each coil of the slinky ultimately returns to its original position. In a water wave, each molecule of the water ultimately returns to its original position. And in a stadium wave, each fan in the bleacher ultimately returns to its original position. It is for this reason, that a wave is said to involve the movement of a disturbance without the movement of matter. The particles of the medium (water molecules, slinky coils, stadium fans) simply vibrate about a fixed position as the pattern of the disturbance moves from one location to another location.
Waves are said to be an energy transport phenomenon. As a disturbance moves through a medium from one particle to its adjacent particle, energy is being transported from one end of the medium to the other. In a slinky wave, a person imparts energy to the first coil by doing work upon it. The first coil receives a large amount of energy that it subsequently transfers to the second coil. When the first coil returns to its original position, it possesses the same amount of energy as it had before it was displaced. The first coil transferred its energy to the second coil. The second coil then has a large amount of energy that it subsequently transfers to the third coil. When the second coil returns to its original position, it possesses the same amount of energy as it had before it was displaced. The third coil has received the energy of the second coil. This process of energy transfer continues as each coil interacts with its neighbor. In this manner, energy is transported from one end of the slinky to the other, from its source to another location.
This characteristic of a wave as an energy transport phenomenon distinguishes waves from other types of phenomenon. Consider a common phenomenon observed at a softball game - the collision of a bat with a ball. A batter is able to transport energy from her to the softball by means of a bat. The batter applies a force to the bat, thus imparting energy to the bat in the form of kinetic energy. The bat then carries this energy to the softball and transports the energy to the softball upon collision. In this example, a bat is used to transport energy from the player to the softball. However, unlike wave phenomena, this phenomenon involves the transport of matter. The bat must move from its starting location to the contact location in order to transport energy. In a wave phenomenon, energy can move from one location to another, yet the particles of matter in the medium return to their fixed position. A wave transports its energy without transporting matter.
Waves are seen to move through an ocean or lake; yet the water always returns to its rest position. Energy is transported through the medium, yet the water molecules are not transported. Proof of this is the fact that there is still water in the middle of the ocean. The water has not moved from the middle of the ocean to the shore. If we were to observe a gull or duck at rest on the water, it would merely bob up-and-down in a somewhat circular fashion as the disturbance moves through the water. The gull or duck always returns to its original position. The gull or duck is not transported to the shore because the water on which it rests is not transported to the shore. In a water wave, energy is transported without the transport of water.
The same thing can be said about a stadium wave. In a stadium wave, the fans do not get out of their seats and walk around the stadium. We all recognize that it would be silly (and embarrassing) for any fan to even contemplate such a thought. In a stadium wave, each fan rises up and returns to the original seat. The disturbance moves through the stadium, yet the fans are not transported. Waves involve the transport of energy without the transport of matter.
In conclusion, a wave can be described as a disturbance that travels through a medium, transporting energy from one location (its source) to another location without transporting matter. Each individual particle of the medium is temporarily displaced and then returns to its original equilibrium positioned.
1. TRUE or FALSE:
In order for John to hear Jill, air molecules must move from the lips of Jill to the ears of John.
2. Curly and Moe are conducting a wave experiment using a slinky. Curly introduces a disturbance into the slinky by giving it a quick back and forth jerk. Moe places his cheek (facial) at the opposite end of the slinky. Using the terminology of this unit, describe what Moe experiences as the pulse reaches the other end of the slinky.
3. Mac and Tosh are experimenting with pulses on a rope. They vibrate an end up and down to create the pulse and observe it moving from end to end. How does the position of a point on the rope, before the pulse comes, compare to the position after the pulse has passed?
4. Minute after minute, hour after hour, day after day, ocean waves continue to splash onto the shore. Explain why the beach is not completely submerged and why the middle of the ocean has not yet been depleted of its water supply.
5. A medium is able to transport a wave from one location to another because the particles of the medium are ____.
a. frictionless
b. isolated from one anotherc. able to interactd. very light******************************************************
False - A sound wave involves the movement of energy from one location to another, not the movement of material. The air molecules are the particles of the medium, and they are only temporarily displaced, always returning to their original position.
When the slinky reaches the end of the slinky and hits Moe in the cheek, Moe experiences a pulse of energy. The energy originated on Curly's end and is transported through the medium to Moe's end. The last particle on Moe's end transports that energy to Moe's cheek.
The point returns to its original position. Waves (and pulses) do not permanently displace particles from their rest position.
Ocean waves do not transport water. An ocean wave could not bring a single drop of water from the middle of the ocean to shore. Ocean waves can only bring energy to the shore; the particles of the medium (water) simply oscillate about their fixed position. As such, water does not pile up on the beach.
Answer: C
For a wave to be transmitted through a medium, the individual particles of the medium must be able to interact so that they can exert a push and/or pull on each other; this is the mechanism by which disturbances are transmitted through a medium.



 How the Chemical Reaction Takes Place in a Battery
How the Chemical Reaction Takes Place in a Battery












